- Home
- >>
- Summary Notes
- >>
- Clinical Practice
- >>
- Blood Gas Reading
Introduction
Arterial Blood Gas (ABG) readings are a very useful, quick investigation that are able to tell you:
- The baseline status of a patient
- If interventions are helping the patient (repeats)
- A differential with the ABG pattern the patient has
It is therefore a key skill to be able to interpret these readings.
There are up to 5 components of any ABG reading- the following are their reference ranges:
(THEY DON’T NEED TO BE MEMORISED- YOU JUST HAVE TO KNOW WHAT THEY MEAN!)
- pH: 7.35 – 7.45
- PaCO2: 4.7 – 6.0 kPa || 35.2 – 45 mmHg
- PaO2: 11 – 13 kPa || 82.5 – 97.5 mmHg
- Base excess (BE): -2 to +2 mmol/L (Remember Base=Alkali)
- HCO3– (bicarbonate): 22 – 26 mEq/L (A chemical base- is the same thing as BE)
Oxygen
Before interpretation- it is important to look at and immediately act on a decrease in PaO2 -HYPOXAEMIA. As this is the most immediately life threatening condition to the patient.
A patient is considered hypoxaemic when PaO2<10 kPa.
A patient is in respiratory failure when this hypoxaemia is severe at <8 kPa.
This is covered further in: Non-Specific Conditions.
Key Facts
There are key facts that should be known that will make interpretation more intuitive:
- There are two homeostatic components that change the acid-base balance in the body:
- Respiratory – via the lungs
- CO2 dissolves in water (and blood) to make things more acidic – (think of pollution causing acid rain)
- The lungs affect CO2 concentrations in the blood – can make the blood more or less acidic by breathing at different rates
- Metabolic via the kidneys
- HCO3/BE (bicarbonate) dissolves in water (and blood) to make things more alkaline/basic
- The kidneys affect the concentrations of bicarbonate- so can make the blood more or less alkaline by getting rid of bicarbonate to urine at different rates.
- Respiratory – via the lungs
- These components are like two opposite ends of a set of scales. – If one side changes – to maintain pH balance the other side will try to change in the other way.
Interpretation
There are 3 steps in interpretation:
1- What is pH saying?
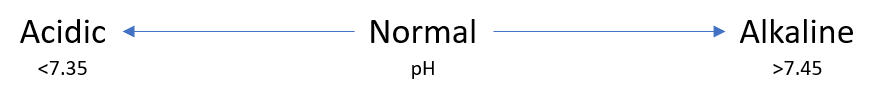
2- Is anything driving a change in pH?
There are 2 causes- with their own list of differential diagnoses behind them:
- Respiratory -Change in PaCO2
- If ↓pH and PaCO2↑ -RESPIRATORY ACIDOSIS [Caused by anything ↓respiratory rate]
- Respiratory depression (drugs such as opiates)
- Asthma
- COPD
- Iatrogenic (not enough mechanical ventilation)
- If ↑pH and PaCO2↓ – RESPIRATORY ALKALOSIS [Caused by anything ↑respiratory rate]
- Panic Attacks – (hyperventilation)
- Pain
- Pulmonary Embolism (PE)
- Pneumothorax
- Iatrogenic (too much mechanical ventilation)
- If ↓pH and PaCO2↑ -RESPIRATORY ACIDOSIS [Caused by anything ↓respiratory rate]
- Metabolic– Change in HCO3/BE
- If If ↓pH and HCO3/BE↓ -METABOLIC ACIDOSIS
- Diabetic ketoacidosis
- Lactic acidosis
- Renal failure
- Diarrhoea
- If ↑pH and HCO3/BE ↑ -METABOLIC ALKALOSIS
- Vomiting
- Loop and Thiazide Diuretics
- Heart Failure
- Nephrotic Syndrome
- If If ↓pH and HCO3/BE↓ -METABOLIC ACIDOSIS
3- Is there anything driving compensation – trying to make the pH normal again?
If a component is deranged- then the opposite component will try to compensate to get the blood back to a normal pH:
- METABOLIC COMPENSATION:
- In a respiratory acidosis – pH= low, PaCO2=high – to compensate the kidneys would increase HCO3/BE in blood to increase pH back to normal.
- In a respiratory alkalosis- pH=high, PaCO2=low – to compensate the kidneys would do the opposite to decrease pH back to normal.
- RESPIRATORY COMPENSATION
- In a metabolic acidosis- pH=low, HCO3/BE=low – to compensate the lungs would work harder to blow off CO2 in blood to increase pH back to normal.
- In a metabolic alkalosis-pH= high, HCO3/BE=high- to compensate the lungs would do the opposite to increase CO2 in blood to decrease pH back to normal.
Worked Example
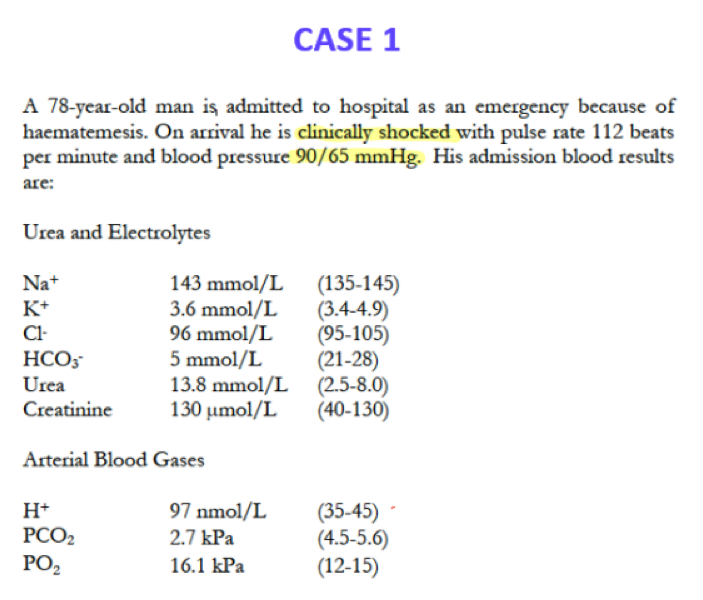
Often, there are other electrolytes given, but focus on what you know. – pH is essentially a measure of H+ concentration, a lower value meaning a higher concentration of H+.
In this scenario there is blood loss via vomit (haematemesis) which is causing the decrease in HCO3 causing a clear acidic pH in the blood – A METABOLIC ACIDOSIS
Additionally due to the decrease in PCO2 there is a PARTIAL RESPIRATORY COMPENSATION where there is an increased respiratory rate to blow off more CO2 (explaining the additional increase in O2) in an attempt to decrease the H+ to normal.